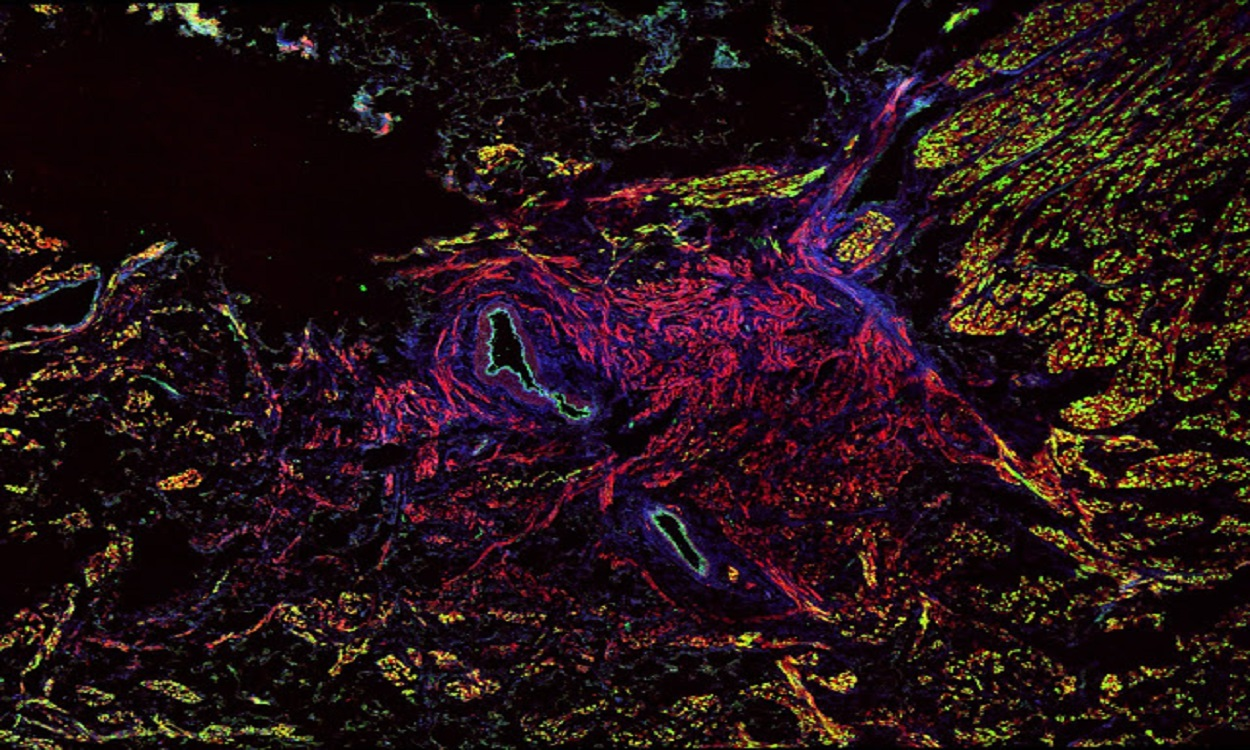
Researchers at The Ohio State University Wexner Medical Center have discovered the human sinoatrial node is hardwired with a backup system. In the groundbreaking study, they have found that three diverse regions of pacemakers acting as batteries and up to five conduction pathways that act as wires to connect the signal to the atria.
AUG 03, 2017 | BY RATNESHWAR THAKUR
A heartbeat begins when the sinoatrial node fires off an electrical impulse that disperses across the atria, the blood-collecting chambers. The sinoatrial node (SAN) is the primary pacemaker of the human heart and responsible for initiating and regulating cardiac rhythm.
Since the discovery of the SAN by Keith and Flack in 1907, multiple studies have investigated the SAN structure and its role in the formation and regulation of sinus rhythm in human and different animal hearts. However, until now, scientists didn’t know for sure how the human SAN protected the heart’s rhythm or how the system failed.
Due to the methodological limitations of studying the heart in patients, the current perception of the SAN is a very simplistic.
The study is published in the Journal “Science Translational Medicine.”
It has been challenging to study human SAN pacemaker complex as it is a heterogeneous 3D structure that lies within the atrial wall, and clinical electrode recordings are limited to only surface atrial activation. Furthermore, the human SAN differs greatly from well-studied experimental animal models.
To overcome these challenges, Dr. Fedorov and his team integrated the high-resolution optical mapping with 3D structural reconstruction and molecular mapping, to reveal the functional, structural, and molecular characteristics underlying robustness of the human SAN pacemaker and conduction complex.
Defects in the pacemaker can lead to heart rhythm disorders that are commonly treated by doctors through implantation of electronic pacemaker devices.
Dr. Fedorov’s team believes that their research may contribute significantly to the development of novel treatments for human SAN dysfunction and cardiac arrhythmias.
“Specifically, our study is providing the foundation for seeking out ways to improve or restore SAN function by targeted treatments in patients with impaired SAN and thus avoiding an electronic pacemaker implant. Our hope is that someday, electronic pacemaker implants could be obsolete,” said Dr. Vadim Fedorov.
Story source:
THE HAWK
